Injectable drugs, a long winding road
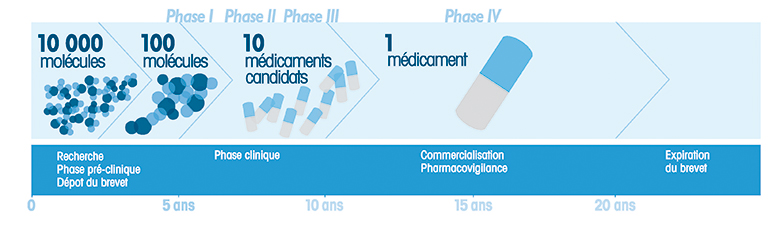
From research into molecules through to sale of the end product, 12 or 13 years are required to develop an injectable drug. The “gestation” of medicines in bags involves the same four phases as standard drugs, but with a few more specific constraints. Spotlight on a road fraught with pitfalls.
Once exploratory research and preclinical testing of the molecules have been completed, Phase I clinical trials start on healthy volunteers. They serve to determine the maximum dose tolerated by man, as well as the administration method. Phase II evaluates the dose-effects relationship, defines the drug dosage and detects short-term adverse effects. It is also the starting point for development of the primary packaging. In order to be compatible with the drug and to guarantee perfect stability, the material used for the future bag has to conform to numerous parameters. The aim is to avoid any interaction with the product that may pose a threat to the quality of the injectable and hence to the patient’s safety.
Key point: the raw material of the bag. This material undergoes a study of extractables and leachables, then an assessment of toxicity and risks. At this stage, Technoflex R&D also verifies that compatibility between bag and spout is optimal (diameter, aseptic filling or not, etc.).
Factors other than the composition of the drug can affect the stability of the product in contact with the container. This is the case of ambient temperature and humidity. The stability study carried out also has to take account of low temperatures as well as the freezing/thawing cycles, particularly for biotechnologies and blood derivatives. For certain preparations1 it is also indispensable to consider the effects of exposure to light.
Phase III could be called the “comparative testing” phase. The drug’s properties are compared with a placebo or an existing drug. Only if an acceptable benefit-risk ratio is proven can a MA (Market Authorisation) be awarded. At this stage of testing, the injectable’s primary packaging is ready: polypropylene, EVA or PVC depending on the nature of the drug. The final phase starts when the drug is marketed: this is pharmacovigilance. Knowledge of the drug in actual conditions of use is furthered in this way.
The goal of therapeutic research is to develop high-quality, efficient injectable drugs. It is a long, complex process. The expertise of all the main players is required for innovative treatments to emerge.
- Extractables are compounds of plastics that can be extracted by solvents with physical and chemical properties that differ under aggressive conditions.
- Leachables refer to compounds that can be released by plastics into the pharmaceutical products under normal conditions of use.
Sylvie Ponlot