LASA drugs under high surveillance
 The greater the number of steps involved in preparing a drug, the higher the risks. This is the case for injectables which have a wide range of applications. They are used in anesthesia, intensive care, chemotherapy, nutrition, and antibiotic therapy. Taking the administration route used into account, they require care and vigilance through to administration and demand increased surveillance when the prescription involves drugs referred to as Look-Alike and/or Sound-Alike drugs (LASA).
The greater the number of steps involved in preparing a drug, the higher the risks. This is the case for injectables which have a wide range of applications. They are used in anesthesia, intensive care, chemotherapy, nutrition, and antibiotic therapy. Taking the administration route used into account, they require care and vigilance through to administration and demand increased surveillance when the prescription involves drugs referred to as Look-Alike and/or Sound-Alike drugs (LASA).
At the end of the 1960s, only around ten products were affected by such similarities, whether visual (Look-Alike) or phonetic (Sound-Alike). This resemblance is found in the name, the shape or the packaging. Today, the number of pairs or trios of LASA drugs is estimated at more than 3,000. The main factor responsible? The decline in patents. It has triggered the arrival of many generic drugs with similar names and the same active principle. This is particularly the case for anti-infective agents. The confusion causes many accidents with potentially fatal consequences, as they sometimes involve dangerous substances such as powerful tranquillizers or anticancer drugs.
LASA drugs alone account for a quarter of the drug-related errors reported in the United States. To reduce their impact on patient safety, multiple measures have been undertaken. First of all, the first critical step of reading the prescription when it is handwritten, is gradually being computerized. It contains precise, important information about the drug and its preparation. Concentration, dose, diluent, speed of perfusion, all information essential to avoid confusion, to safeguard reconstitution in the hospital pharmacy and administration to the patient. Still in the hospital environment, numerous recommendations have been drafted. Among other things, they recommend not buying several generic drugs with the same active principle, as well as better organization of injectables storage by separating dangerous products from the rest or additional labelling to better identify them.
Another concrete preventive action relates to the drug typography used on primary and secondary packaging. Alternate cases, lower case and capitals, have been used to better identify the different syllables. The use of capital letters has noticeably improved the legibility of the product name. More recently in the United States, the Institute for Safe Medication Practices (ISMP) has gone further. It now recommends enhancing visibility by incorporating bold characters: LOOK-Alike & SOUND-Alike.
Regulations and packaging, solutions exist
Many government bodies such as the ANSM or the FDA have published a list of all LASA drugs. It is updated regularly, but it is difficult, even impossible, to standardize these lists at international level as many pharmaceutical products have different names in the different countries where they are marketed. Still from a regulatory perspective, it is possible to act upstream, particularly before granting a marketing authorization. When filing an application, a commercial name which is not similar to a product that already exists could be required under penalty of refusal of approval.
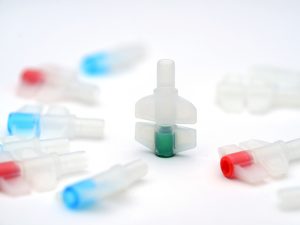 Regarding packaging, another solution is conceivable and is easily carried out: the attribution of a color code per product category that would be visible on the primary and secondary packaging. In the case of injectable drugs packaged in flexible bags, administration ports such as twist-off or luer-lock ports could display specific colors (blue, red, green, etc.) according to product. For the other formulations, the color would appear on the secondary packaging and the product label.
Regarding packaging, another solution is conceivable and is easily carried out: the attribution of a color code per product category that would be visible on the primary and secondary packaging. In the case of injectable drugs packaged in flexible bags, administration ports such as twist-off or luer-lock ports could display specific colors (blue, red, green, etc.) according to product. For the other formulations, the color would appear on the secondary packaging and the product label.
Many avenues for improvement may therefore be envisaged to reduce the risk of errors associated with Look-Alike and Sound-Alike drugs. But to implement them, only international collaboration between the pharmaceutical industry, packaging manufacturers, regulatory bodies and final users would remove the remaining barriers.
Sylvie Ponlot



















