PP bags
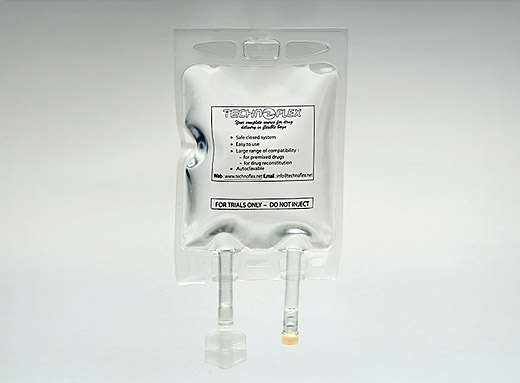
PP bags for drug supplementation
The proven and recognized chemical inertness of polypropylene Inerta® bags enables medicinal solutions to be packaged in reliable, tried and tested containers.
PP bags for drug supplementation are compliant with European and American pharmaceutical requirements. They meet client requirements in terms of the container-content interactions, as tested during the stability studies necessary for the marketing of molecules such as antibiotics, analgesics, cardiology products, contrast media, anaesthetics etc.
For more information, contact the Technoflex Sales Department
For an inquiry in the USA: usa.sales@technoflex.net
Properties
- Polypropylene Inerta® film*
- DMF n° 19057
- USP class 6 and ISO 10993 compliance
- No plasticisers (DEHP-free)
- Excellent chemical inertness
- Easy and secure use thanks to Twist-Off system
- Closed system
- Terminal sterilisation 125°C
- Good oxygen and water vapour barrier
* Other polypropylene films available on request
Uses
- Anaesthetics
- Analgesics
- Antibiots
- Cardiology products
Technoflex R&D meet your specific needs
| Volume | Tubes or boat port | Connectors | Code | Dimensions | Packing |
|---|---|---|---|---|---|
| 50 ml | 2 | Twist Off Spike Port TP 823 | IX 0050 2T | 12.7 x 9.0 | 800 |
| 100 ml | 2 | Twist Off Spike Port TP 823 | IX 0100 2T | 16.0 x 10.0 | 800 |
| 250 ml | 2 | Twist Off Spike Port TP 823 | IX 0250 2T | 16.0 x 12.5 | 500 |
| 500 ml | 2 | Twist Off Spike Port TP 823 | IX 0500 2T | 22.5 x 13.5 | 500 |
| 1 000 ml | 2 | Twist Off Spike Port TP 823 | IX 1000 2T | 31.9 x 13.5 | 400 |
| 2 000 ml | 2 | Twist Off Spike Port TP 823 | IX 2000 2T | 31.1 x 25.0 | 250 |
| 3 000 ml | 2 | Twist Off Spike Port TP 823 | IX 3000 2T | 35.2 x 25.0 | 250 |
| 5 000 ml | 2 | Twist Off Spike Port TP 823 | IX 5000 2T | 42.5 x 28.5 | 150 |















